The European Union and GIZ Ghana are providing technical support to the Food and Drugs Authority (FDA in the implementation of a comprehensive institutional and technical strengthening programme.
The programme is intended to deliver various capacity-building activities in the areas of marketing authorization, inspection of good manufacturing practice, testing, and release of vaccines, in readiness for effective regulation of vaccine manufacturing in Ghana.
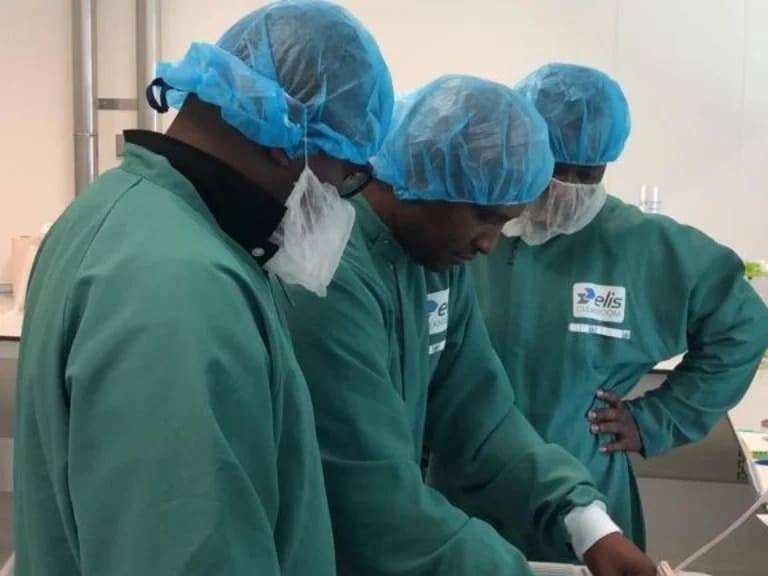
As part of this technical support, a team of FDA inspectors drawn from directorates responsible for Inspections, Quality Control, Marketing Authorization, and Industrial Support embarked on a customized and intensive Hands-on Vaccine Good Manufacturing Practice (GMP) training programme.
The three-week training programme was designed and delivered by the Biotech Training Facility, one of the leading training institutes on vaccines and biopharmaceuticals in Europe located in Leiden Bioscience Park, Netherlands.
The key objective of the training was to equip the officers of the FDA with the requisite technical knowledge required for regulatory oversight of vaccines in general and specifically for locally manufactured vaccines to meet international standards of safety, quality, and efficacy.
The first week of the training programme focused on foundational topics such as quality management systems, facility design for vaccine manufacturing, good documentation practices, data integrity and the qualification of equipment, facilities, and utilities for the production of bulk substances, upstream and downstream processing in vaccines among others.
The second part of the training was delivered as an in-person programme at the Biotech Training Facility in Leiden, Netherlands.

The world-class training centre is furnished with state-of-the-art equipment, clean rooms, laboratories and technical areas operated by highly experienced staff bio-manufacturing.

Training areas covered during these hands-on sessions included critical steps involving upstream fermentation cell culture and downstream purification processes, pathways for vaccine development, harvesting, and clarification technologies for downstream purification.

The team was also involved in various processes such as affinity chromatography, ion exchange, tangential flow filtration, diafiltration filtration, nano-filtration, viral clearance steps and formulations for biosafety and quality control of vaccines.


Trainees had the opportunity to interact and learn from SMEs on utilities such as Heating, Ventilation and Air Conditioning Systems and Bulk Water purification Systems for vaccine manufacturing and other related areas such as commissioning, qualification, and maintenance of these critical utilities.
The benefits of the hands-on Vaccine GMP training programme include, but are not limited to:
- Helping the realization of the vision of the President – Ghana becoming a vaccine manufacturing hub
- Ensuring more officers are well trained and prepared to conduct thorough Good Manufacturing Practice Inspections when facilities are established for local production as well as inspection of foreign vaccine manufacturing facilities.
- Guaranteed access to safe and efficacious vaccines which will protect the public and enhance the fight against infectious diseases in the country.
- Knowledge transfer to other officers internally, and
- Strengthen the capacity of personnel in the Industrial Support Department to assist local manufacturers to comply with cGMP requirements in the near future.
This comprehensive training programme will in the long term enhance the capacity of officers of the Authority in providing the needed regulatory oversight to support the vision of Ghana becoming a vaccine manufacturing hub in Africa.
Source: FDA


Comments